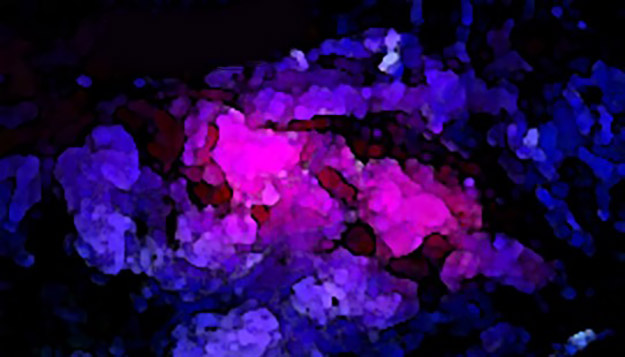
Approved in June 2017 by the U.S. Food and Drug Administration and launched commercially in-country in October 2018, 5-Aminolevulinic Acid (also known as 5-ALA, and composed of aminolevulinic acid hydrochloride) is an optical imaging agent for use in patients with high-grade gliomas. Nicknamed the “pink drink,” 5-ALA is a surgical…...
Free Cancer Screening Guidelines