Free Cancer Screening Guidelines
The World’s First Oncolytic Virus Drug was Launched to Treat Malignant Brain Tumor GBM
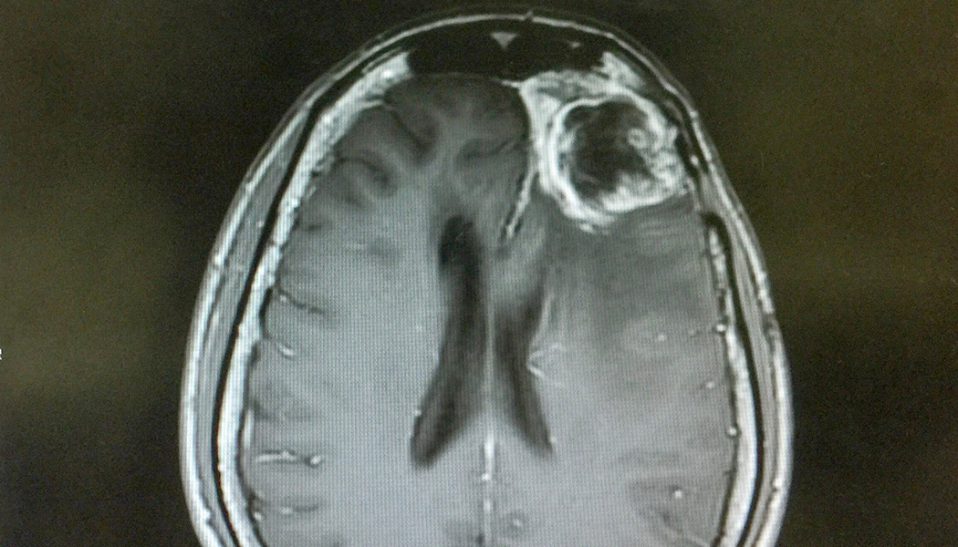
Glioblastoma multiforme (GBM) is the most aggressive malignant brain tumor in adults, with a median survival of only about ten months. Unlike low-grade gliomas (grades I and II), which grow slowly, high-grade gliomas (grades III and IV) grow much faster and can spread to other parts of the brain, resulting…...
Origin Cells of Glioblastoma May Have Been Identified
In what could be a major breakthrough, scientists at the Korea Advanced Institute of Science and Technology (KAIST) in South Korea claim to have identified the cellular origin of glioblastoma, a fast-growing type of central nervous system tumor that forms from supportive tissue of the brain and spinal cord. It…...