Credentials
Professor and Interim Chair of the Department of Molecular, Cell and Cancer Biology,
Inaugural Our Danny Cancer Fund Chair in Biomedical Research,
Leader of the Cancer Genetics Program,
UMass Cancer Center
University of Massachusetts Chang Medical School
Worcester, MA
Research Projects
The cancer-causing protein MYC is abnormally activated (or “turned on”) in about 70% of cancers, where it plays a central role in driving tumor formation and growth. Interestingly, tumors become dependent on MYC for their continued growth, and therefore inhibiting MYC would be a powerful approach to treat many types of cancers.
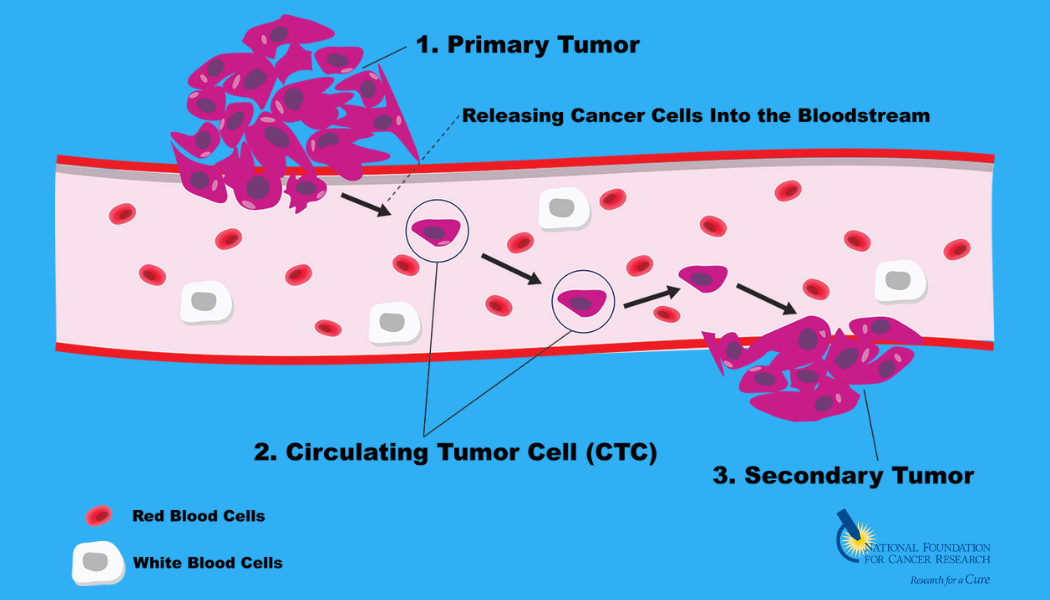
However, developing conventional drugs that target MYC has been a major challenge due to its “undruggable” protein structure. Dr. Kelliher’s research group will use a new compound that targets an important protein required for MYC activation, thereby effectively turning MYC off. Using the MYC-dependent blood cancer, multiple myeloma, as a model system, Dr. Green demonstrated the compound slows growth of human multiple myeloma cells cultured in the lab and reduces growth of multiple myeloma tumors in complex models.
As a next step toward developing this compound as a new cancer drug, Dr. Kelliher’s team will test its ability to stop growth of circulating tumor cells (CTCs) isolated directly from multiple myeloma patients using the advanced cancer cell detection technology.
IMPACT
The results of Dr. Kelliher’s study will significantly impact the cancer research field and benefit cancer patients by testing the efficacy of a promising new approach for treating multiple myeloma and other MYC-dependent cancers, including breast, colorectal, liver and prostate cancers.
Background
Michelle A. Kelliher, Ph.D., has a long-standing interest in hematopoietic malignancies. Her laboratory has investigated how oncogenes such as TAL1, LMO and NOTCH1 mediate leukemic transformation, and identified novel drug targets to resensitize relapsed pediatric leukemia patients to glucocorticoid therapy. Her lab was the first to develop patient-derived xenografts from pediatric leukemia patients to perform preclinical trials of novel targeted therapies. Her lab has also made major contributions to cell death regulation with the development of RIPK1 knockout, conditional and kinase inactive mice.
Michelle A. Kelliher received her Ph.D. at Tufts University in 1991 and completed post-doctoral research at Harvard Medical School in the Department of Genetics. She became faculty member of the University of Massachusetts in 1998.
Dr. Kelliher has received numerous award: Leukemia and Lymphoma Society Special Fellow Award (1998), Sidney Kimmel Cancer Scholar Award (2000-2002), Leukemia and Lymphoma Society Award (2003-2008), and Leukemia and Lymphoma Society Stohlman Scholar Award (2008). Dr. Kelliher is a member of the Leukemia and Lymphoma Society Career Development Review Panel, a member of the NIH Cancer Genetics Study Section (2008-2012) as well as a reviewer for the Charles A. King Trust Postdoctoral Fellowship Program and the Hood Foundation Research Award.